Discovery Biology
Pharmacokinetics
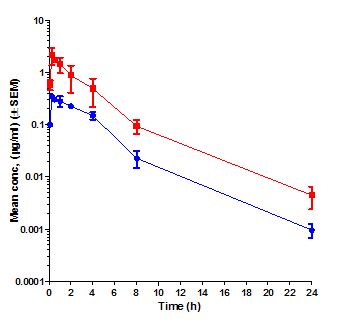
Pharmacokinetics (PK) is the study of time course of drug’s or compound’s concentration in the body (what the body does to the drug or compound). The concentration profile of a compound or drug in the body is the result of the processes of Absorption (A), Distribution (D), Metabolism (M) and Elimination (E) or ADME. Most importantly the concentration of a compound in the body is linked to its efficacy or toxicity. Hence, characterizing the PK of a compound early on in discovery programs helps in evaluation of its drug-like properties.
Fundamental PK parameters such as Volume of Distribution at steady state (Vss), Systemic Clearance, elimination half-life, the Area Under the Concentration Time curve in blood (AUC), Peak concentration in blood (Cmax), time to peak concentration in blood (Tmax), and Oral Bioavailability help in understanding the compound’s ability to show efficacy or toxicity in animals and human beings. Determination of PK parameters in preclinical studies help in optimizing molecules for efficacy and safety, progression to crucial pharmacology and toxicology studies, in prediction of human PK using Allometric scaling and Physiologically Based Pharmacokinetic (PBPK) and in Pharmacokinetic/Pharmacodynamic (PK/PD) modelling.
Dose Linearity: Single And Repeat Dose
Routes: Oral and Parenteral
Single Dose PK: Basic PK Characterization
Repeated Dose PK: Steady State, Accumulation
Dose Escalation PK
Dose Linearity: Single And Repeat Dose
Tissue Distrubution
Bioavailability (BA) and Bioequivalence (BE)
PK Analysis (Winnonlin)
PK Parameters, Modeling and Simulation of PK
Prediction of PK in Human: Allometry, FTIM Dose
PK/PD
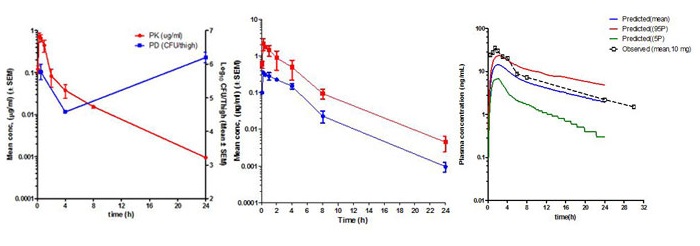
The relationships between the time course of drug or compound concentration (Pharmacokinetics, PK) and the onset, intensity and duration of pharmacological effect (Pharmacodynamics, PD) form the basis for evaluation of the drug’s efficacy and potency (referred to as Quantitative Pharmacology) in preclinical animal models of disease and in human patients.
Determining PK/PD relationships for compounds early on in discovery programs help in a)progression of the optimum compound for advanced testing; b)prediction of efficacy and potency based on in vitro and PK data; c)prediction of efficacy in humans by extrapolation of preclinical PK/PD models; and most importantly in setting dose, regimen and duration in pre-clinical pharmacology studies and in humans.

Establishment of Relationships between Pharmacokinetics (PK) and Pharmacodynamics (PD)
Time Course Of Dose-concentration
- Response Studies To Enable PK/PD Modelling
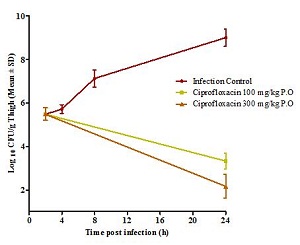
Dose Fractionation Studies To Identify PK/PD Index Of Efficacy In Mouse And Rat Models Of Infection
- Thigh Infection
- Lung Infection
- Septicemia
- UTI, cUTI
- IAI

Modelling and Simulation
The in vivo Pharmacokinetics (PK) and Pharmacodynamics (PD) of a compound or drug can be represented as mathematical models. Developing PK, PD or PK/PD models help in simulations of what-if scenarios such as prediction of quantitative pharmacological effect when a compound is given as single, two or three doses in a day, concentration-time profiles for different doses and regimens, time needed for complete efficacy.
Such simulations help in designing optimal experiments, selection of time points for collection of data, prediction of effect in long duration studies thus avoiding time consuming studies, and prediction of PK and PD profiles in human populations. Modelling and Simulation (M&S) is a powerful tool in drug discovery and development and is being increasingly sought by the regulatory authorities for decision making.
Modelling PK: NCA, Compartment, PBPK models
PK/PD Modelling
Simulation of "what-if" scenarios with PK, PD and PK/PD models
Prediction of Human PK : Allometry, PBPK
Pharmacology
In the current era of innovation in drug discovery, biotechnology, medical devices or natural products, a key factor in evaluation of a newly designed molecule or product is to obtain robust and quality data from optimally designed experiments.
We at Oncogenix endeavour to meet these criteria. Oncogenix offers wide range of animal disease models covering a variety of critical therapeutic areas such as infection & AMR, oncology, metabolic disorder, wound healing, dermatology, inflammation & pain & neurology. We actively consult with our partners in selection of appropriate models as well as establish and validate new models.

Animal Models of Infection
Mouse
- Complicated Urinary Tract Infection (cUTI)
- Urinary Tract Infection
- Thigh Infection in Neutropenic Mice with Gm +ve & Gm –ve Pathogens
- Neutropenic Thigh Infection Model: Dose Fractionation to Identify PK/PD Index of Efficacy
Rat
- Complicated Urinary Tract Infection (cUTI)
- Urinary Tract Infection
- Thigh Infection in Neutropenic & Non-Neutropenic Rat with Gm +ve & Gm –ve Pathogens
- Neutropenic Rat Lung Infection Model with Epithelial Lining Fluid PK
Rabbit & Guinea Pig
- Keratitis
- Rhinosinusitis
- Acute Otitis Media
- Neutropenic Guinea Pig Model of Systemic Candidiasis
Animal Models of Metabolic Disorder
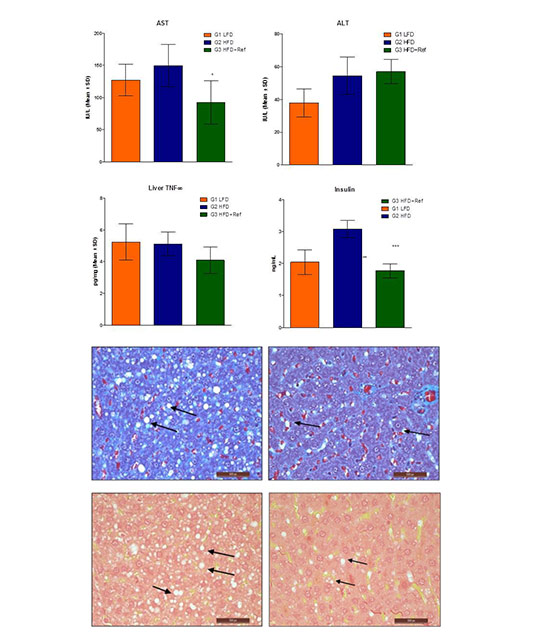
- ❯NASH Model in Rat
- ❯Adriamycin Induced Chronic Nephropathy (CKD) in Mice
- ❯Folic Acid induced Acute Kidney Injury (AKI) in Mice
- ❯Cisplatin Induced Acute Kidney Injury (AKI) in Mice
- ❯Genetic Models – Zucker Obese Rat (Zucker fa/fa), db/db Mice and Zucker Diabetic Fatty (ZDF)
- ❯Diet Induced - Obesity Using C57BL/6 Mice (DIO)
- ❯HFD/STZ (Low Dose) Induced Type 2 Diabetes in Rats
- ❯STZ Induced Diabetes Type1
- ❯STZ + Nicotinamide induced Diabetes Type 2
- ❯Carbon Tetrachloride (CCl4) Induced Liver Fibrosis in Mice
- ❯Ischemic Reperfusion Kidney Injury in Rat (IRI)
- ❯Triton Induced Hyperlipidemia
- ❯Cholesterol Induced Hypercholesterolemia
- ❯OGTT/IGTT Assay
- ❯Gentamycin Induced Nephro-toxicity
- ❯Sodium Oxalate Induced Urolithiasis
- ❯Osteoporosis Model in Rat
Animal Models of Dermatology
- ❯Alopecia Models (Testosterone, DHT) in Mice
- ❯Psoriasis Model in Mice
- ❯Atopic Dermatitis Model in Mice
- ❯Vitiligo Model in Guinea Pigs
- ❯Acne Model in Mice
- ❯Inflammation Model in Mice & Rat
- ❯Anti-wrinkle Activity in Mice

Animal Models of Auto Immune Disorder

- ❯Arthritis - Collagen induced Arthritis (CIA) (Rat & Mouse), Adjuvant Induced Arthritis (AIA) (Rat)
- ❯Imiquimod Induced Psoriasis in Mice
- ❯Experimental Autoimmune Encephalomyelitis (EAE) in Mice
- ❯Colitis – Dextran Sulphate Sodium (DSS) Induced (Ulcerative Colitis, Mouse), Trinitrobenzene Sulphonate (TNBS) Induced (Crohn's Disease, Rat)
- ❯Hypersensitivity Type 1 – Ovalbumin Induced Dermatitis (Mouse), Oxazolone Induced Dermatitis (Mouse)
Animal Models of Wound Healing

- ❯Excision Wound Healing in Rat
- ❯Excision Wound Healing in Diabetic Induced Rat
- ❯Excision Wound Infection in Rat
- ❯Excision Diabetic Wound Infection in Rat
- ❯Burn Wound Healing in Rat
- ❯Burn Wound Infection
Animal Models of Inflammation & Pain
- ❯Carageenan Induced Paw Edema in Rat
- ❯LPS Induced Pulmonary Inflammation (Rat)
- ❯Bleomycin Induced Pulmonary Fibrosis (Rat)
- ❯Oxazolone / Xylene / Arachidonic Acid / Croton Oil Induced Ear Edema
- ❯Cotton Pellet Induced Granuloma
- ❯Chemically Induced Pain Model - Acetic Acid Induced Writhing (Rat), Formalin Induced Tonic Pain (Rat & Mouse)
- ❯Hot and Cold Plate Method
- ❯Tail-Flick Test
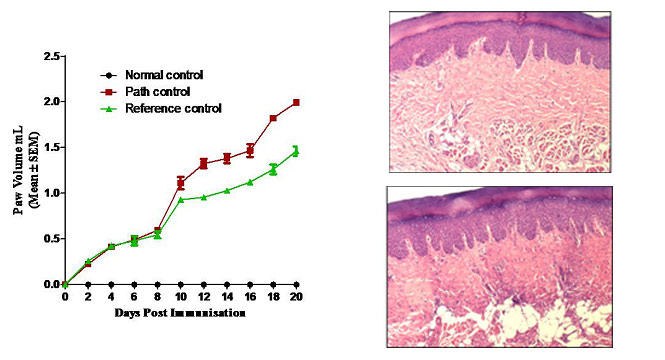
Animal Models of Neurology
- ❯Epilepsy – Isoniazide (INH) Induced, Picrotoxin Induced, Maximum Electro Shock (MES) Induced Convulsion
- ❯Parkinsonism – MPTP Induced
- ❯Depression – Forced Swim Test, Tail Suspension Test
- ❯Antipsychotic – Inhibition of Amphetamine Stereotypy in Rats, Inhibition of Apomorphine Climbing in Mice
- ❯Nootropics – Morris Water Maze, T-Maze, Y-Maze
- ❯Anxiety – Elevated Plus Maze, Light-Dark Model, Open Field Test
- ❯Muscle Coordination – Rotarod Test, Grip Strength Test, Dexamethasone Induced Cachexia
- ❯Alzheimer’s Disease
- ❯LPS Induced Neuro Inflammation

Animal Models of Ulcer
- ❯Pylorus Ligation in Rat
- ❯Indomethacin Induced Gastric Ulcer
- ❯Stress Induced Ulcer (Cold, Heat and Restraint Stress)
- ❯Ethanol-Induced Gastric Ulcer in Rat
Oncology
Cancer is one of the most difficult diseases to treat, with high mortality rates of 92.3% within five years of diagnosis. A major challenge encountered in cancer drug discovery and development is the high attrition rate of development candidates in clinical trials due to lack of efficacy. This lack of efficacy in clinical studies has been attributed to low accuracy of preclinical models to predict efficacy of compounds in the clinic. As a result, preclinical models with high predictive value for efficacy are urgently required.
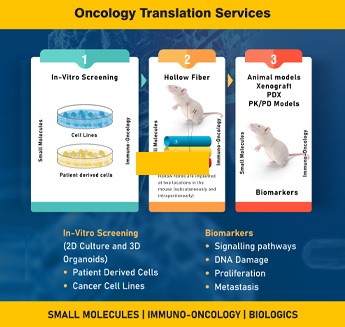
Discover Oncogenix Oncology, where groundbreaking cancer drug discovery and development thrives through advanced preclinical models and unparalleled expertise. Our experienced Oncology team offers a comprehensive range of innovative solutions, including 2D/3D Organoid models, Hollow Fiber models, iPSC screening platform, as well as PDX and GEM models. These models are meticulously designed to replicate and explore intricate tumor environments with precision.
Integrated pharmacology models further enhance our capabilities by incorporating biomarkers, ensuring robust translational insights from preclinical stages to clinical development. At Oncogenix, we specialize in bridging the gap between discovery and application, providing tailored approaches and comprehensive support to accelerate your oncology research.
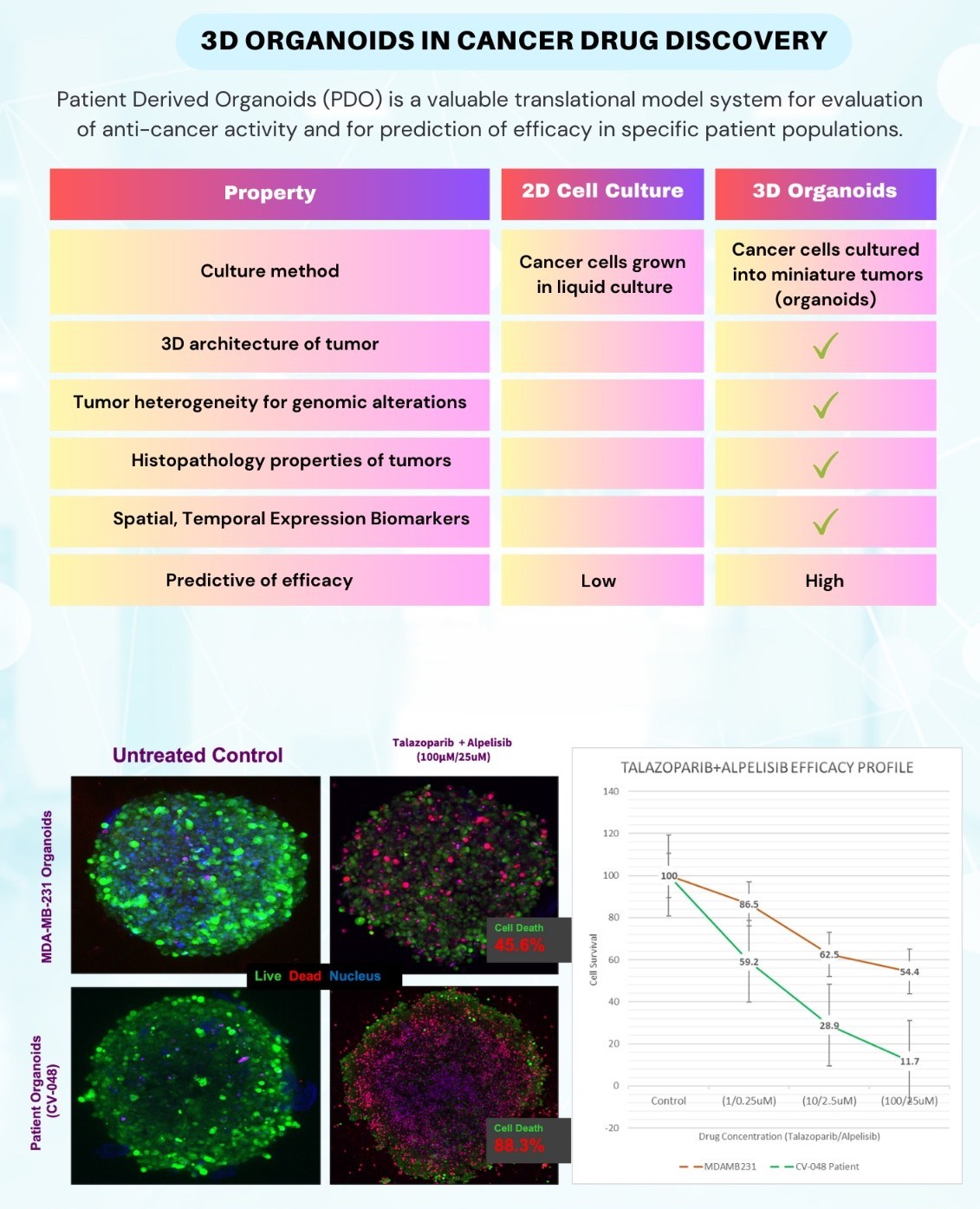
Organoid Models For Cancer Drug Discovery
The 3D Organoid model was developed to overcome the shortcomings of the 2D method. Here the cancer cells derived from human cancers are grown into organoids which have the characteristics of tumors in terms of architecture (histology) and genetic heterogeneity. They have been shown to maintain the characteristics of the tumor taken from the patient.
The patient derived organoids (PDOs) are valuable tools to evaluate the anti-cancer activity of clinical drug candidates in drug discovery programs in Cancer. The results obtained from the organoid screen are more predictive of efficacy in patients as they take into account the accessibility of drug to tumor cells across barriers and the heterogeneity of mutations.
Patient Derived Organoids (PDO) significantly improve the translational success for anti- cancer therapeutics in clinical trials.
Advantages:
- Demonstration of Early Proof of Concept:Testing of Lead Compounds in PDOs expressing the pharmacological (drug) target help to establish proof of concept early in program and increase confidence for clinical efficacy. They also help in identification of Biomarkers for efficacy that can be applied in clinical trials.
- Identification of target populations for Clinical Trials:At the nomination stage, Clinical Drug Candidates can be screened against a bank of PDOs, expressing the drug target, to identify specific patient molecular types in which they are maximally effective. This will enable selection of appropriate patients in clinical trials and increase the confidence for high clinical response.
- Identification of Cancer Indications:Drug Candidates can be screened against PDOs from different cancers expressing the same abnormally expressed drug target to identify cancer indications for clinical trials.
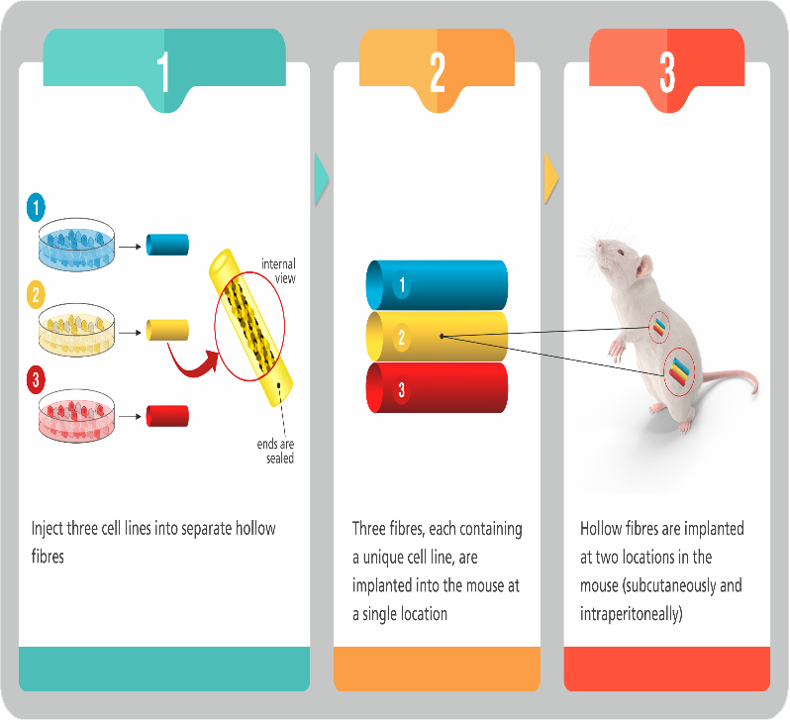
Hollow Fibre Models
Hollow Fiber Models form the link between in vitro efficacy and in vivo efficacy in Xenograft models of cancer. They can be effectively employed to rapidly screen many molecules for preliminary evaluation of Pharmacokinetics and Pharmacodynamics.
Advantages:
- Rapid in vivo screening of anti-cancer molecules (few days)
- PK and PD information obtained from the same animal
- Prioritization of molecules for confirmatory studies in xenograft models
Animal Models
XENOGRAFT
- ❯Brain
- ❯Breast
- ❯Cervical
- ❯Colon
- ❯Hepatocellular
- ❯Gastric
- ❯Leukaemia
- ❯Lung
- ❯Melanoma
- ❯Ovarian
- ❯Pancreatic
- ❯Prostate
- ❯Renal
ORTHOTOPIC
- ❯Leukaemia / Lymphoma
- ❯Breast
- ❯Liver
- ❯Stomach
- ❯Kidney
- ❯Prostate
- ❯Skin